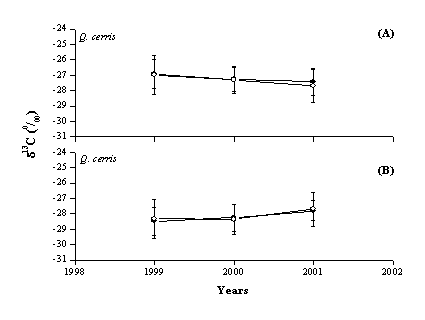Comparing carbon isotope composition of bulk wood and holocellulose from Quercus cerris, Fraxinus ornus and Pinus radiata tree rings
Forest@ - Journal of Silviculture and Forest Ecology, Volume 1, Pages 51-57 (2004)
doi: https://doi.org/10.3832/efor0217-0010051
Published: Oct 12, 2004 - Copyright © 2004 SISEF
Research Articles
Abstract
Tree-ring δ13C is widely employed in ecophysiological studies, because it represents an integrated proxy of the ratio between photosynthesis (A) and stomatal conductance (g), which expresses the intrinsic water use efficiency (iWUE), strongly affected by the environmental conditions experienced by the plant during its life span. Tree-ring δ13C also reflects long term variations of atmospheric CO2 concentration and of its carbon isotope composition, partly due to increasing anthropogenic emissions. Carbon isotope abundances in tree rings can be assessed on bulk wood as well as on wood biochemical components, wich show different δ13C values because of secondary discrimination during biosynthesis. We present the results of a comparison between δ13C values of bulk wood and holocellulose samples obtained from the last three (1999, 2000 and 2001) annual growth rings of two hardwood (Quercus cerris L. and Fraxinus ornus L. and one conifer (Pinus radiata D. Don, species. We found that 13C values differed significantly among tree species, both in the case of holocellulose and bulk wood, but only in the case of P. radiata bulk wood samples tend to provide more negative δ13C values than holocellulose, as reported in the literature. We suggest that, at least for the two hardwood species studied, bulk wood is a suitable material to work with for δ13C assessment, whilst in P. radiata holocellulose could provide a more stable and reliable index, when studying plant ecophysiological responses to changing environmental conditions.
Keywords
Carbon isotope composition, Tree ring, Bulk wood, Holocellulose
Introduction
Tree-ring carbon stable isotope ratio (13C/12C) depends on discrimination against the heavier carbon isotope, 13C, during photosynthesis (CO2 diffusion through the stomata and carbon fixation in the chloroplast) and during secondary biochemical reactions (translocation and differentiation of photosynthates).
According to the model by Farquhar et al. ([9]), in C3 plants carbon isotope discrimination strongly depends on CO2 concentration in leaf intercellular spaces (ci), which is further regulated by stomatal conductance (g) and by photosynthetic efficiency (A). Several environmental factors affect stomatal conductance and, therefore, plant carbon stable isotope composition.
Tree-ring δ13C is widely employed in ecophysiological studies, because it represents an integrated proxy of the ratio between photosynthesis (A) and stomatal conductance (g), which expresses the intrinsic water use efficiency (iWUE) strongly affected by the environmental conditions experienced by the plant during time. Tree-ring δ13C also reflects long term variations of atmospheric CO2 concentration and of its carbon isotope composition, partly due to increasing anthropogenic emissions ([28], [10]).
Carbon stable isotope composition in tree rings can be assessed on bulk wood as well as on wood biochemical components, such as lignin, holocellulose and α-cellulose. Different wood components show different δ13C values: for instance, cellulose is 13C enriched by 1-2 ‰ relative to whole wood, while lignin is depleted by 2-6 ‰ relative to whole wood and by 4-7 ‰ relative to cellulose ([1], [6]). Depletion observed for secondary plant products, especially lignins, aromatic compounds, flavonoids originating from the pathway of shikimic acid, is due to the kinetic isotope effect of the pyruvate dehydrogenase reaction ([20], [14]).
For assessing tree ring δ13C, analysis of cellulose extracts could theoretically be preferred because i) cellulose does not migrate from the tree ring in which it was formed and ii) less reactions (and fractionations) occur during its synthesis from photosynthates, in comparison with biosynthesis of lignin and other wood compounds. Bulk wood analyses may lead to δ13C values more affected by lignin, resins and highly mobile carbon reserves, such as carbohydrates, which tend to migrate between adjacent tree rings and whose amount may vary widely between sapwood and heartwood ([30], [16], [27]). Furthermore, the ratio of lignin and cellulose concentrations is quite variable in bulk wood, thus, when measuring its δ13C, it would represent the mixing ratio of lignin and cellulose and not the water use efficiency of the plant studied ([32]). On the other hand, cellulose extraction is a time-consuming process, requiring the employment of several reagents and may represent a bottle-neck when trying to analyse long-time tree ring series.
Borella et al. ([3]) provided an in-depth analysis of the uncertainties arising when measuring tree ring δ13C and, in particular, tried answering the question if cellulose extraction is needed for δ13C assessment; they showed good correlations (r2 > 0.8) between δ13C of α-cellulose and bulk wood for hardwood species (oak and beech), concluding that “wood δ13C is as good climate proxy as cellulose”. On the other hand, they warned that extractives should at least be removed from conifer wood, as it can have variable and high resin content.
In this paper we present the results of a comparison between δ13C values of bulk wood and holocellulose obtained from the last three (1999, 2000 and 2001) annual growth rings of two hardwood (Quercus cerris L, Fraxinus ornus L.) and one conifer (Pinus radiata D.Don) species, growing in the same forest.
In the literature many methods for cellulose extraction have been described (see among others [26], [15], [29], [5], [23], [18], [21], [4]), but the “Jaime-Wise” method ([15]) and its subsequent modifications ([16], [27], [17]) are used in most cases.
The main goal of the present paper is to test for differences between δ13C values of bulk wood and holocellulose, in order to assess which one can be a more suitable proxy of water use efficiency of trees and, therefore, of short term changing environmental conditions.
Materials and Methods
Plant material was collected in December 2001, on Q. cerris (n = 44), F. ornus (n = 14) and P. radiata (n = 6) trees growing in the Stio forest (Southern Italy, province of Salerno, 40°18’32“N, 15°15’07” E, 700 m a.s.l.), at an altitude of about 700 m a.s.l. Climate is Mediterranean, humid type; mean temperature is 13°C and annual precipitation 1200 mm.
The studied forest is a 22-24 year old P. radiata D. Don plantation wich extends over 106 ha. Within the forest two stands have been selected, homogeneous according to all environmental conditions but for light regime. The stands have been identified as follows: lightly thinned stand (one over three rows of trees) with natural regeneration of Q. cerris (n = 16 in S2); strongly thinned stand (one over two rows of trees) with natural regeneration of Q. cerris (n = 15 in S3) and F. ornus (n = 14 in S3). As a control a coppice of native Quercus species (n = 13 in S1) has been selected too, growing in the same area.
In the case of hardwoods, trees were cut at their base and a 3 cm thick wood disc was sawn off from the lowermost stem portion; in the case of pine, a wood core was extracted by means of an increment borer from the NW facing side of the stem at breast height. Q. cerris and F. ornus were 6-7 years old saplings, while P. radiata 22-24 years old trees. Tree-ring width was quite regular in each studied species for all the rings sampled.
Taken soon to the laboratory, samples were oven dried at 60 °C for 24 hours. Afterwards, thin shavings were obtained with a razor blade from each of the last three (1999, 2000 and 2001) annual growth rings (whole ring for hardwoods and NW facing section of the ring for the conifer); material was further reduced to 0.2 mm particles using a micro-gear miller and a ceramic mortar. Of each wood sample: i) one half underwent direct analysis of carbon isotope abundances into a Finnigan Delta Plus (Finnigan, Bremen, Germany) mass spectrometer (bulk wood samples, WS); ii) the other half underwent holocellulose (hereafter indicated as cellulose) extraction according to the Jaime-Wise method ([15]), modified by Leavitt & Danzer ([16]); afterwards, holocellulose was analysed into the same Finnigan Delta Plus mass spectrometer (cellulose samples, CS). Cellulose extraction was carried out in two steps: i) removal of sugars and lipids by means of a soxhlet apparatus, keeping the samples 48 hours in a 2:1 solution of toluene and ethanol and 48 hours in pure ethanol, ii) removal of lignin, keeping the samples 4 days in a solution of sodium chlorite (NaClO2) and glacial acetic acid (CH3 COOH) at 70 °C (bleaching). Before bleaching, samples were taken out from the soxhlet to dry and boiled in a beaker with deionised water for 6 hours to remove hydro-soluble sugars. The level of the solution during bleaching was kept constant refilling the beaker when needed; at the beginning of each day of bleaching samples were accurately rinsed in deionised water and the solution was replaced by a newly prepared one.
All statistics were carried out using the SPSS statistical package (SPSS Inc. 1989-1999).
Results and discussion
According to one-way ANOVA, tree-ring carbon isotope composition (δ13C) did not differ among annual growth rings in any of the studied tree species, as well as holocellulose (CS) or bulk wood samples (WS) were considered (Tab. 1).
Tab. 1 - Differences among adjacent tree ring δ13C values according to one-way ANOVA.
| Species | Bulk wood | Holocellulose | ||||
|---|---|---|---|---|---|---|
| F | df | P | F | df | P | |
| Quercus cerris | 0.381 | 131 | 0.684 | 0.056 | 143 | 0.945 |
| Fraxinus ornus | 0.307 | 47 | 0.737 | 0.129 | 49 | 0.879 |
| Pinus radiata | 0.616 | 17 | 0.501 | 0.707 | 15 | 0.513 |
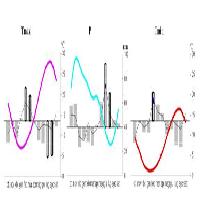
In the present case of study site climatic conditions did not show significant differences among years (data not shown): mean annual temperature was slightly above the average value and water was not a limiting factor since precipitation amounts were larger than the average value. Also, light environment in each stand did not change significantly during the short study period. Therefore, the absence of significant variations in δ13C among years could be attributed to steady climatic and environmental conditions experienced by the trees during the different years, as showed also by ring widths (data not shown). In fact, yearly tree ring δ13C values did not differ among them both in the case of BW and CS (Fig. 1). δ13C values of adjacent tree rings from the same plant may thus be averaged in case of short term physiological studies, according to previous findings ([3]), for reducing the effect of lignin migration and of translocation of previous year photosynthates during earlywood construction.
Fig. 1 - Tree ring δ13C values of bulk wood (•) and holocellulose (º) according to year in S1 (A), S2 (B) and S3 (C) for all the studied species.
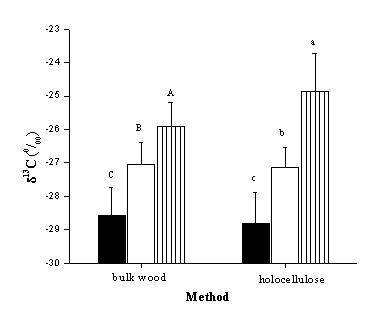
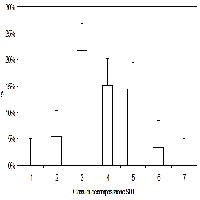
Values of δ13C differed significantly among tree species, both in the case of CS and WS. In particular, Q. cerris showed the lowest (more negative) isotopic composition (Fig. 2); carbon isotope discrimination is considered a good proxy of water-use efficiency ([8]) and the observed differences in isotopic composition could likely be attributed to contrasting ecophysiological characteristics between the studied species ([30], [7]) as well as to varying lignin to cellulose ratios among years and species. In fact, the two species show different stomatal behaviours as discussed in the literature: Q. cerris exhibits a lower stomatal control than F. ornus ([31]) when experiencing water stress ([22]).
Fig. 2 - Comparison between species; δ13C values (mean ± SD) of bulk wood and cellulose of Q. cerris (left column), F. ornus (central column) and P. radiata (right column). Different capital (bulk wood) and lower case (holocellulose) letters indicate significant differences (P < 0.05) among species according to S-N-K multiple comparison test; δ13C values from different growth rings in the same plant have been considered as replicates.
At least in part, the δ13C values in Q. cerris and F. ornus saplings may also be affected by a “juvenile effect” ([12], [2]), which consists of low and/or steadily increasing values of wood and cellulose δ13C during the early stages of tree growth. This “juvenile effect” has been attributed to a different microenvironment during the phase of seedling establishment, to uptake of13 C depleted respired CO2 from the canopy floor and from the soil, or to age-related physiological factors ([13], [11], [19], [2]).
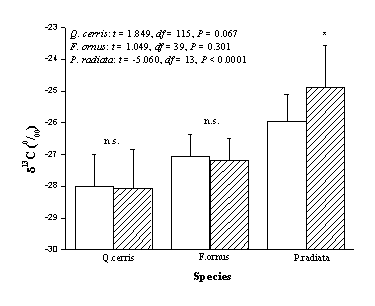
In both Q. cerris and F. ornus no significant difference in δ13C was found between CS and WS (according to paired-samples t-test), despite holocellulose was found to be depleted in13 C by 0.04 ‰ in oak and by 0.09 ‰ in ash, relative to bulk wood (Fig. 3). Holocellulose δ13C could have been affected by hemicellulose, whose content is greater in juvenile wood ([24]) and which is depleted in13 C with respect to cellulose ([1], [21]). On the other hand, in P. radiata δ13 C values were significantly more negative in WS than in CS.
Fig. 3 - Comparison between methods; δ13C values (mean ± SD) of bulk wood (left column) and holocellulose (right column) of Q. cerris, F. ornus and P. radiata. n.s. means not significant whilst * indicates significant differences, according to paired sample t-test; δ13C values from different growth rings in the same plant have been considered as replicates.
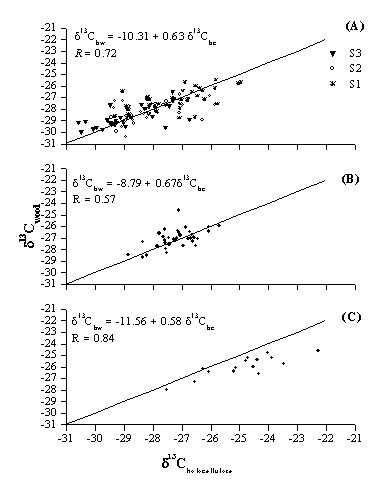
Indeed, looking at the relationships between individual δ13C values in CS and WS samples, it can be observed that in Q. cerris and F. ornus data points are rather well scattered around the one-to-one line, with no apparent systematic deviation (Fig. 4A and B), whilst in the case of P. radiata WS tends to provide more negative δ13C values (Fig. 4C). Besides, the large range of variation of δ13C values in Q. cerris for both CS (5.6‰) and WS (4.8‰) depends on the fact that trees were sampled in three different stands with changing light conditions; therefore δ13C values varied among stands depending on the characteristics of the above canopy cover (pine versus native oak coppice, data not shown). In fact, according to One way Anova, δ13C values in Q. cerris differed significantly among stands, for both WS (F = 26.936, df = 129, P < 0.00001) and CS (F = 18.247, df = 111, P < 0.00001), with S1 showing higher δ13C values relative to S2 and S3.
Fig. 4 - Relationship between δ13C values of bulk wood and holocellulose in Q. cerris (A), F. ornus (B) and P. radiata (C); the line represents the 1:1 relationship.
With respect to the relationship reported by Borella et al. ([3]) (Fig. 4A) for oak wood, we have found a lower correlation coefficient between δ13C of CS and WS, due to larger scatter in our data, but a closer agreement to the one-to-one relationship. The slope of regression between holocellulose and bulk wood is always smaller than 1, showing that the isotopic variations in CS are larger than the variations in WS. This result could indicate that some interesting climatic or environmental signal may be preserved in δ13C of holocellulose while it can be partially lost or masked in δ13C of bulk wood. This can be due to the fact that, unlike wood lipids and other compounds, holocellulose derives directly from the primary photosynthates ([14]); thus, its carbon isotope composition could better reflect the climatic and environmental conditions which affected the photosynthetic pathway.
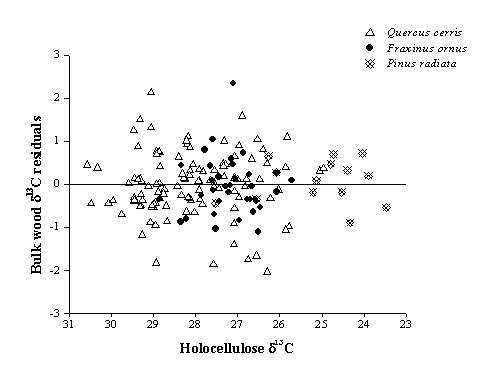
On the other hand, it is worth noting that regression residuals are unevenly distributed, i.e. irregularly scattered, around the zero value (Fig. 5), thus suggesting caution in the use of a linear regression model.
We may conclude that in Q. cerris and F. ornus, wood carbon isotopic composition is mainly affected by cellulose δ13C and bulk wood seems a suitable material to work with when studying plant ecophysiological response to changing environmental conditions. Only under conditions which may favour rapid polysaccharides degradation, as may occur with wood material suitable for paleoclimatic studies, extraction of lignin may be recommended ([25]). As for P. radiata, our results suggest that δ13C of bulk wood could deviate significantly from δ13C of holocellulose, possibly as an effect of secondary compounds, such as oleoresins, whose amount can vary from year to year and lignin, which tend to migrate among as well as within tree rings. In this case, cellulose δ13C could represent a more stable and reliable index compared to bulk wood.
Acknowledgements
Research work supported by the Italian National Project COFIN-2003 “Interventi selvicolturali e basi ecofisiologiche della rinaturalizzazione di rimboschimenti”, entitled to A.S. (Project leader O. Ciancio) and by funds from “Dottorato di ricerca in arboricoltura da legno”, University of Basilicata. We thank Marco Borghetti and one anonymous reviewer for helpful comments on the manuscript.
References
CrossRef | Google Scholar
Online | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
Google Scholar
Google Scholar
Google Scholar
Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
Online | Google Scholar
Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
Online | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
Google Scholar
Google Scholar
CrossRef | Google Scholar
Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
CrossRef | Google Scholar
Google Scholar
CrossRef | Google Scholar