Is Tuber brumale a threat to T. melanosporum and T. aestivum plantations?
iForest - Biogeosciences and Forestry, Volume 11, Issue 6, Pages 775-780 (2018)
doi: https://doi.org/10.3832/ifor2785-011
Published: Nov 28, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
True truffles in the genus Tuber are the most valuable ectomycorrhizal fungi and their cultivation has become widespread around the world. Competition with other ectomycorrhizal fungi and especially with undesired Tuber species, like T. brumale, can threaten the success of a truffle plantation. In this work, the competitiveness of T. brumale towards T. melanosporum and T. aestivum was assessed in a 14 year-old plantation carried out planting seedlings inoculated with these three truffle species in adjacent plots. Analyses of both truffle ectomycorrhizas and extra-radical mycelium were carried out in the transects separating the T. brumale plot from T. melanosporum and T. aestivum plots. The results confirm the competitiveness of T. brumale against T. aestivum and T. melanosporum due to its major ability to colonize the soil around its ectomycorrhizas. However, its competitiveness is limited to the transect areas and it was never found inside T. melanosporum plot. These results remark that, in presence of optimal conditions for T. melanosporum and T. aestivum, the greatest risk of contamination with T. brumale is due to wrong greenhouse activity.
Keywords
Competition, Black Truffles, Extra-Radical Mycelium, Ectomycorrhizas, Species-Specific Primers
Introduction
Ectomycorrhizas (ECMs) are symbiotic associations between fine roots of woody plants and soil fungi. They represent the most frequent root symbioses in boreal, temperate and subtropical forests and woodlands ([37]). Different species of ectomycorrhizal fungi may live together and share the same environment, establishing competition for nutrients/water in the soil and carbon on the host roots ([23]). Competition among these fungi can be highlighted through the analysis of their communities ([24], [33]). Although the first studies on below-ground ectomycorrhizal fungal communities date back to the mid-1990s ([12]), the mechanisms determining competitive outcomes are not yet entirely understood. The full understanding of these mechanisms is crucial because they affect the survival and spreading of ectomycorrhizal fungi, and different plant-fungus pairings can result in notable modifications in performance for both symbionts ([7], [30]). Competition between ectomycorrhizal fungi becomes of practical relevance when a commercially valuable fungus is introduced in the field through inoculated seedlings obtained in greenhouse. ECMs of the introduced fungal species can be replaced by other native ectomycorrhizal fungi on the host roots ([18]) and threaten the success of the plantation.
True truffles in the genus Tuber are the most valuable ectomycorrhizal fungi and their cultivation has become widespread around the world ([43]). The most cultivated Tuber species are the black truffles Tuber melanosporum Vittad., T. aestivum Vittad. and, to a lesser extent, T. brumale Vittad. These species often share the same natural sites and compete for space on the host roots ([18], [11]). Due to the lower value of the ascomata and the high competitiveness, T. brumale has often been considered as a contaminant, able to replace the ECMs of T. melanosporum in greenhouse or in truffle plantations ([29]). Most of the studies on ectomycorrhizal communities of T. melanosporum plantations carried out in Italy, France and Spain report the presence of T. brumale ([13]). It was also found able to compete with, and in some cases to replace, T. melanosporum in New Zealand and Australia where true truffles are not endemic and T. brumale was probably introduced with the inoculum ([17], [26]). ECMs of T. brumale were also identified in T. aestivum plantations ([42], [6]) but competition between these two black truffles has been poorly investigated. Rather, Tuber aestivum was found replacing T. melanosporum in several black truffle plantations around the world ([5], [15], [41], [13]).
Most of the studies focusing on the competition between black truffles only considered the distribution of their ECMs, while the extra-radical mycelium (ERM) has been little considered, as it is a relatively new target of investigation. Recent studies have shown that the use of species-specific primers on DNA extracted from soil is a sensible and reliable method to identify ([44]) or quantify ([19], [32], [16]) the ERM of a Tuber species in soil samples.
In the past, a number of plantations were established in Italy by planting groups of seedlings mycorrhized with different black truffles species in the same field ([3]). This kind of orchard is well suited to study the competitiveness between the different truffle species because they are subjected to the same experimental conditions. In one of these plantations, we aimed to verify the competitiveness of T. brumale towards T. melanosporum and T. aestivum 14 years after planting. Analyses of both truffle ECMs and ERM were carried out in the transects separating T. brumale from T. melanosporum and T. aestivum plots.
Materials and methods
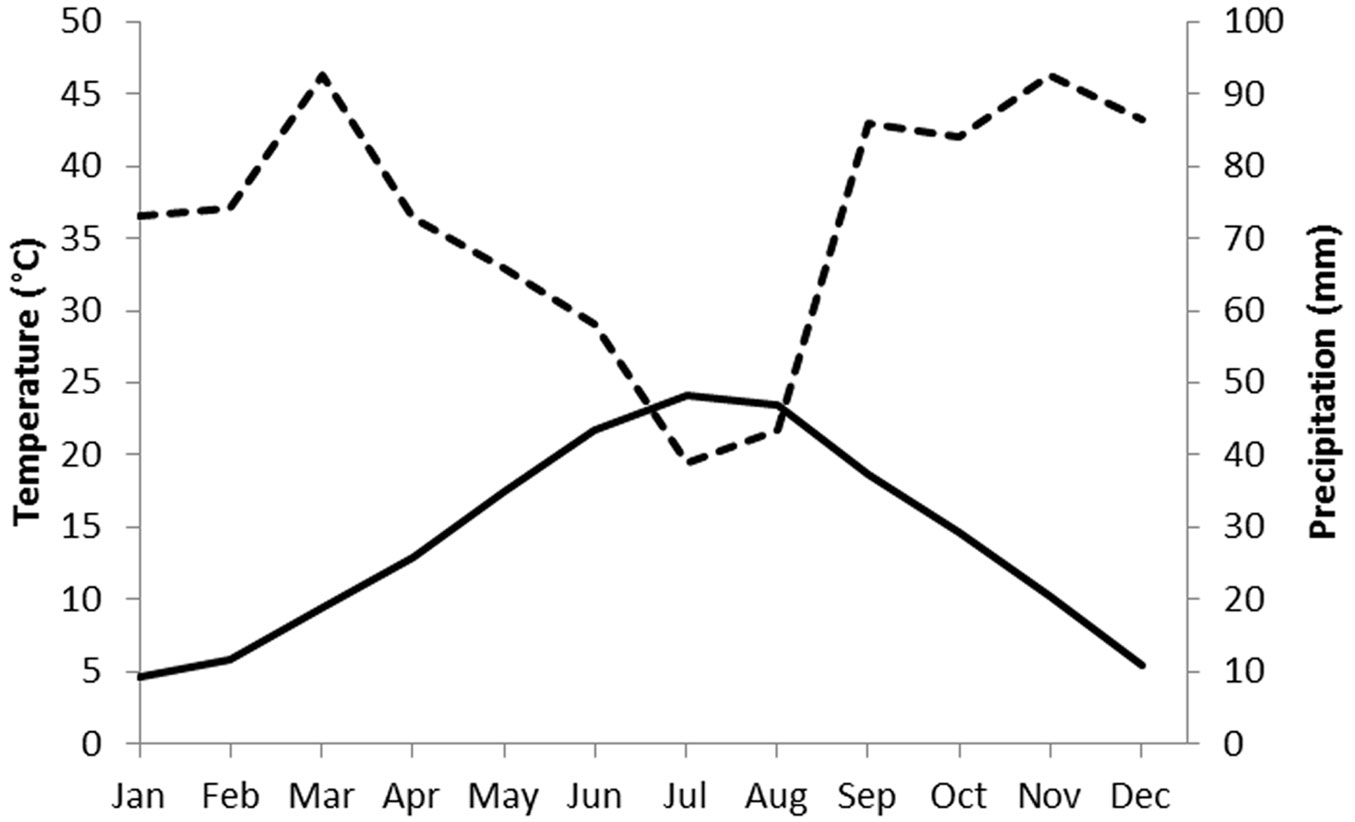
The study site covers an area of 1540 m2, at an altitude of about 80 m a.s.l., in the municipality of Montelabbate (Pesaro-Urbino, Italy - 43.845488 N; 12.788349 E). The truffle orchard under investigation was established in 2002, using plants mycorrhized by spores. The field had been used for arable crops for at least 30 years before planting. The sandy-clay-loam soil (sand 49%, clay 28%, silt 23%) is calcareous (total carbonate 22%) and has a pH of 7.75. The soil organic matter is 1.2%. The climate of this area is characterized by a short summer drought period (Fig. 1); March and November are the wettest months while July (24.1 °C) and January (4.7 °C) are the hottest and coldest months, respectively. The region is suited to T. melanosporum, T. brumale and T. aestivum, which can also naturally occur in the same forest stands.
Fig. 1 - Climatic diagram of Bagnouls-Gaussen for the meteorological station of Montelabbate (Pesaro-Urbino, central Italy). Precipitation and temperature data are for the 2000-2016 period. Monthly temperatures (left axis, °C) are indicated with the solid line, monthly precipitations (right axis, mm) are indicated with the dotted line.
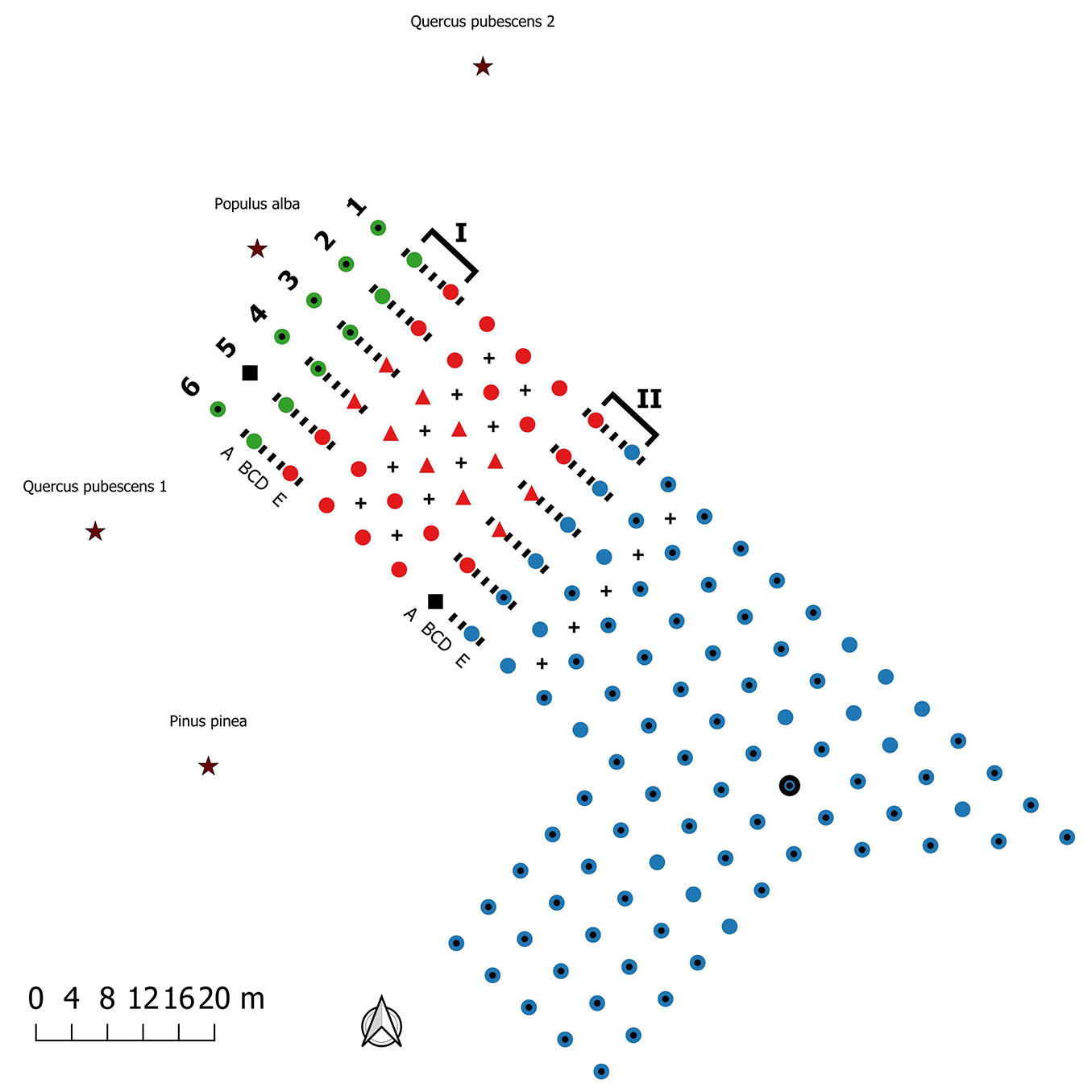
A total of 130 seedlings (120 Quercus pubescens Willd. and 10 Corylus avellana L.) mycorrhized with T. melanosporum, T. brumale or T. aestivum were planted 4 × 4 m apart as shown in Fig. 2. The seedlings were certified by the regional authority ASSAM (Agenzia Servizi Settore Agroalimentare Marche, Osimo AN, Italy) which ensured a minimum mycorrhization of 30% with the target truffle species. At the time of the study, the plantation was grass-covered and the planted trees were 3 to 4 m high with a canopy cover of approximately 50% (Fig. S1 in Supplementary material). Most of the plants mycorrhized with T. melanosporum (79%) and T. aestivum (64%) showed the characteristic brûlé (a vegetation-devoid area around the host plant), while the T. brumale plants did not show it. The plantation was surrounded by mature tree species, some of them ectomycorrhizal such as Q. pubescens, Populus alba L. and Pinus pinea L. The only cultural practices carried out on the truffle orchard after planting were pruning, grass-mowing and irrigation. No tillage was performed. Irrigation was provided during summer by a drip system for the first 3 years after planting and, subsequently, by a sprinkler system every two weeks.
Fig. 2 - Scheme of truffle plantation, transect location and sampling points. Circles indicate Quercus pubescens seedlings; triangles Corylus avellana seedlings; black squares dead plants. Mycorrhized species: Tuber brumale (red), Tuber aestivum (green) Tuber melanosporum (blue). Dotted circles indicate plants with brûlé and the bold circle indicates the only productive plant. Transects are highlighted with a square bracket: (I) aest/brum transect; (II) brum/mela transect. The positions of soil samples collected in each transect are indicated with the “-” symbol. (A-B-C-D-E): soil sample types: A and E were only used for ERM analysis, while B, C and D for both ERM and ECMs analyses. Sampling within plots are indicated with “+” symbol. The black star symbol indicates the adult ectomycorrhizal trees surrounding the plantation: Quercus pubescens 1, Quercus pubescens 2, Populus alba and Pinus pinea.
At the time of the study, only one ascoma of T. melanosporum was collected (February 2016) under a plant mycorrhized with the same truffle species (Fig. 2).
Soil sampling
The soil sampling was carried out in late spring 2016 within two transects between the plots of plants mycorrhized with different Tuber species (Fig. 2): T. aestivum - T. brumale (I: aest/brum) and T. brumale - T. melanosporum (II: brum/mela).
The plants on the margins of the two transects (23 plants in total because one plant died) were selected for soil sampling. Only 3 out of 23 plants on the margin of the two transects shown a brûlé (two in the aest/brum transect and one in the brum/ mela transect). Five sample types (A to E) were collected along each tree row as showed in Fig. 2. Samples were 1 m (B and D) and 2 m (C) far from the trunks into the transect areas, while samples A and E were 1 m far from the trunks into the respective truffle plots. Samples B to D were used for both ERM and ECM analyses while samples A and E were used only for ERM analysis. The 58 soil cores for ERM analysis (5 cores × 6 rows × 2 transects, excluding 2 cores not collected close to the dead plant) were extracted through disposable PVC tubes (30 cm depth and 20 mm in diameter, ~0.1 dm3) to avoid cross contamination. The 35 soil cores for ECM analysis (3 cores × 6 rows × 2 transects, excluding 1 core not collected close to the dead plant) were extracted through a steel soil corer (15 cm depth and 10 cm in diameter, ~1.2 dm3). Five and ten soil samples were also collected within T. melanosporum and T. brumale plots, respectively, to assess the presence/absence of truffle ECMs and ERMs. These samples were taken as described above, following the scheme reported in Fig. 2.
Analysis of truffle ectomycorrhizas
The roots were removed from the soil cores by a 2 mm mesh sieve, washed in sterile water and then examined under a stereomicroscope (×20). The ECMs were assigned to T. aestivum, T. brumale, T. melanosporum or other fungal species on the basis of their morphology ([1]). When morphotyping was not able to distinguish T. brumale and T. melanosporum ECMs (e.g., lacking in cystidia), molecular identification was carried out by applying a direct PCR approach ([21]) using the species-specific primers designed by Rubini et al. ([36]).
The degree of infection was measured by counting the number of living ECMs of each morphotype in all the root samples and expressing the results as a percentage on the total number of ECMs examined.
Analysis of truffle extra-radical mycelia
Soil cores collected for ERM analysis were extracted from the PVC tubes and transferred to 15 ml tubes, taking care to avoid cross contamination. Any root fragments were removed under a stereomicroscope. The soil was stored at -80 °C and lyophilized for three days using a Virtis Benchtop 2K® freeze dryer (SP Industries, Warminster, PA, USA). After drying, soils were ground in a mortar and stored at -20 °C pending further DNA analyses.
The DNA was isolated from the soil samples using the protocol developed by Iotti et al. ([19]), adapted for 0.5 g of soil. Yield and quality of isolated soil DNAs were evaluated by a Nanodrop® ND-1000 (Thermo Fisher Scientific, Waltham, MS, USA).
The presence/absence of the ERM of the target truffle species was verified by using species-specific primers. The primer pair UncI-UncII ([28]) was used to detect T. aestivum while the presence of T. brumale and T. melanosporum was verified by a multiplex PCR approach using ITSB and ITSML as forward primers and ITS4LNG as the sole reverse primer ([36]). PCRs targeting T. brumale and T. melanosporum ERMs were repeated separately with the primer pair combinations ITSB-ITS4LNG and ITSML-ITS4LNG, respectively, to allow the amplification of the low amount of target DNAs that remained undetected by multiplex PCRs.
PCRs were conducted using a T-gradient Thermal Cycler (Biometra, Göttingen, Germany) in a 30 µL mixture volume containing 300 nM each primer, 50 ng DNA, 1 U Ex-Taq® DNA polymerase (Takara Bio Inc., Kusatsu, Japan), 1× Buffer solution, 200 µM dNTP, 4 mM MgCl2, 10 µg of Bovine Serum Albumine.
The cycling parameters were as follows: 3 min of initial denaturation step at 95 °C, followed by 23 cycles of 30 s at 94 °C, 30 s at 63 °C, 45 s at 72 °C and a final extension step at 72 °C for 7 min.
Results
Transect I - aest/brum
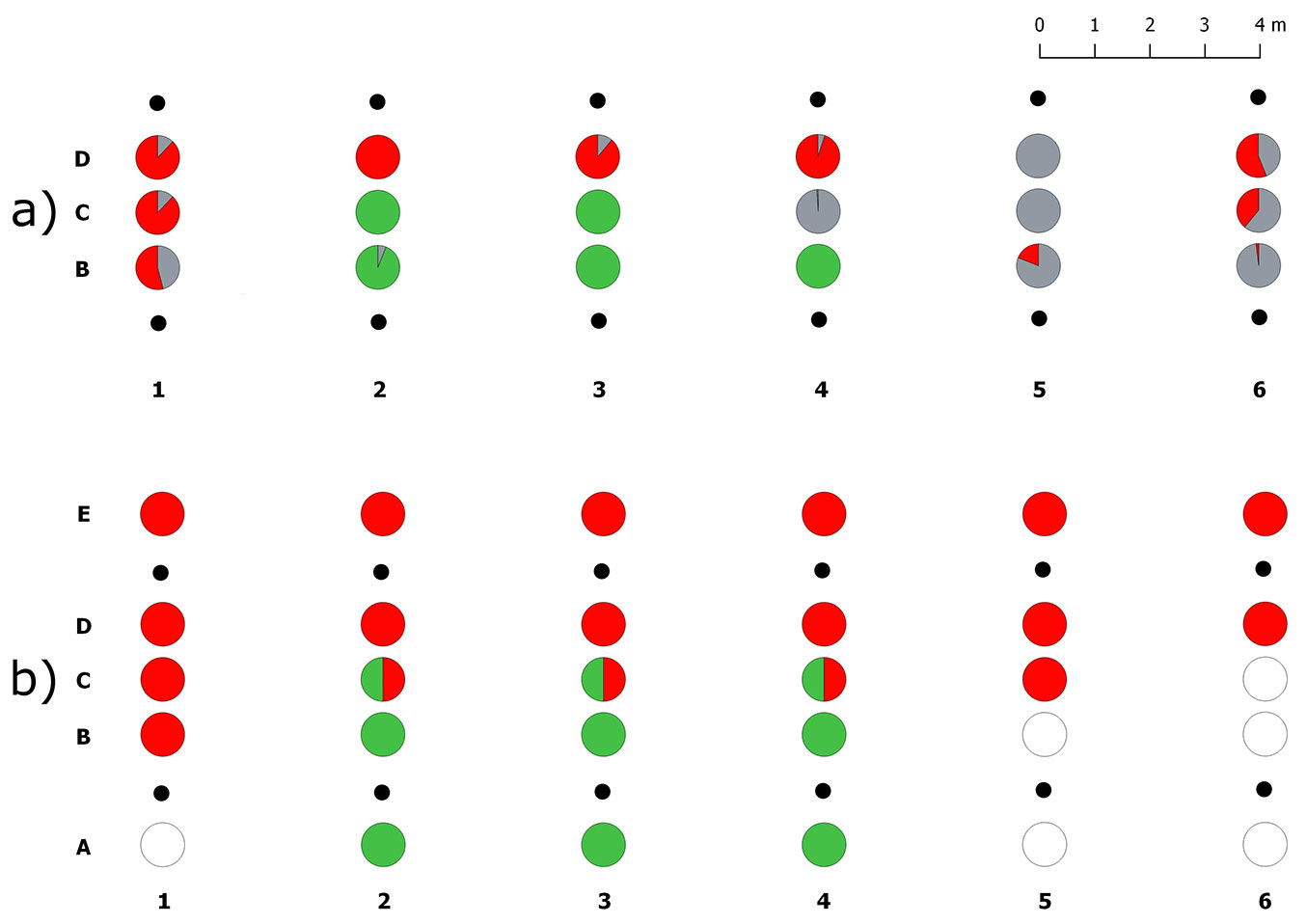
A total of 5115 ECMs were counted in the 18 soil samples collected in the aest/brum transect. More than half the ECMs (60%) belonged to the target Tuber species (32% T. brumale and 28% T. aestivum) whereas other fungi formed only 40% of ECMs (Tab. S1 in Supplementary material). ECMs of T. aestivum and T. brumale were never found mixed in the same soil core (Fig. 3a). Tuber brumale ECMs were abundant in samples D (~70%) although they were also found in samples B, collected 1 m far from the T. aestivum plants (Fig. 3a, Fig. S2). ECMs of T. aestivum were found in only 3 out of 6 plant rows, where it successfully expanded its colonization only as far as the samples collected along the transect midline (samples C, Fig. S2). ECMs of T. melanosporum were never detected in this transect. ECMs formed by other fungi were absent in 5 out of 18 samples where T. aestivum (4 samples) and T. brumale (1 sample) dominated.
Fig. 3 - Spatial distribution of truffle ERMs and ECMs in the transect I (aest/brum). (a) Percentages of ectomycorrhizal colonization of the inoculated truffle species and native ectomycorrhizal fungi in each sample, from B to C; (b) presence/absence of the truffle ERMs in each sample, from A to E. (red): Tuber brumale; (green): Tuber aestivum; (gray): ECMs formed by other fungi; (white): no truffle mycelia; (black dots): host plants.
ERM of the target truffle species was detected in 24 out of the 30 analysed soil samples (Fig. 3b). Tuber brumale and T. aestivum were exclusively found in 15 and 6 samples, respectively, and occurred together in only three samples collected along the transect midline (samples C). In contrast to T. brumale, T. aestivum ERM was never detected in soil samples collected 1 m far from the T. brumale plants (types D and E) and it was completely absent in the whole sample sets (types A to E) of half plant rows. Soil samples collected within the two brûlés in this transect did not have ECMs or ERM of T. brumale.
Transect II - brum/mela
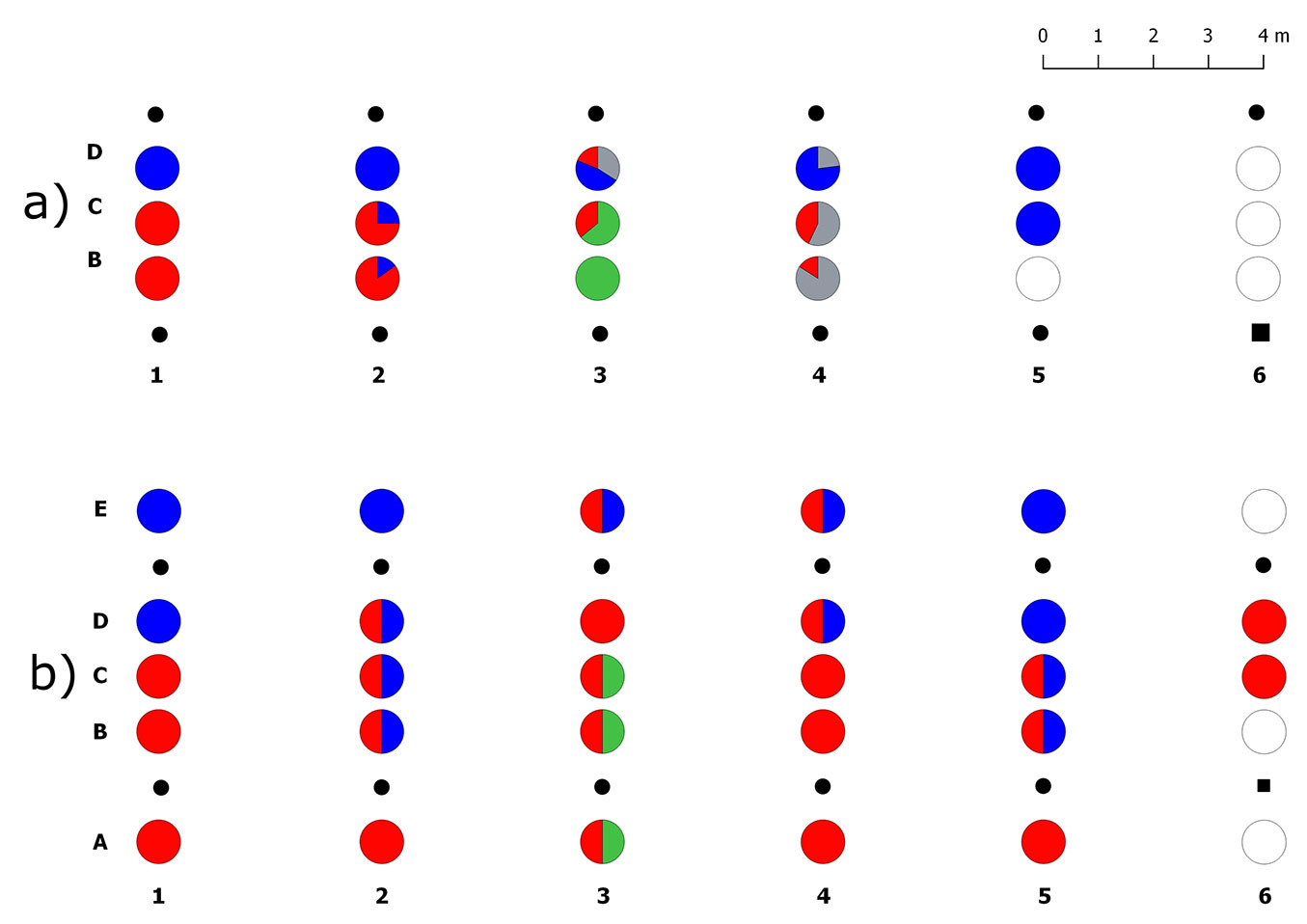
A total of 4310 ECMs were counted in 14 soil samples collected in the brum/mela transect. No roots were found in three soil samples, all collected close to the T. brumale dead plant in row 6. ECMs of the target Tuber species amounted to about 62%, whereas the remaining 38% was formed by other ectomycorrhizal fungi (Tab. S2 in Supplementary material). Tuber brumale dominated the community in this transect with 35% of ECMs, while T. melanosporum reached 27% (Fig. 4a). ECMs of both species showed the same frequency but they co-occurred in only three soil cores. Tuber melanosporum was mainly found in samples collected 1 m far from the trees inoculated with this species (type D), whereas T. brumale was mainly found in samples B (1 m far from its plants) and C (transect midline - Fig. S2 in Supplementary material). ECMs of T. aestivum (26% in total) were also found in two soil cores collected close to a T. brumale inoculated seedling in row 3. One of these samples (type C) showed ECMs of both T. aestivum and T. brumale.
Fig. 4 - Spatial distribution of truffle ERMs and ECMs in the transect II (brum/mela). (a) Percentages of ectomycorrhizal colonization of the inoculated truffle species and native ectomycorrhizal fungi in each sample, from B to C; (b) presence/absence of the truffle ERMs in each sample, from A to E. (red): Tuber brumale; (green): Tuber aestivum; (blue): Tuber melanosporum; (gray): ECMs formed by other fungi; (white): no truffle mycelia; (black dots): host plants; (black square): dead host plant.
ERM of the target truffle species was detected in all the 28 analysed soil samples (Fig. 4b). Tuber brumale DNA was amplified in ~90% of samples including those collected 1 m far from the T. melanosporum plants (types D and E). However, five of these samples had a little amount of T. brumale mycelium with respect to that of T. melanosporum since its DNA was not amplified by multiplex PCR. T. melanosporum ERM was found in 13 soil cores, ~61% of which occurred together with T. brumale. The presence of T. aestivum DNA was detected in three soil cores, in row 3, where its ECMs were also found. Soil samples collected within the brûlé in this transect did not have ECMs or ERM of T. brumale.
No truffle ECMs and ERMs different from the inoculated species were detected in the soil samples collected within T. melanosporum and T. brumale plots.
Discussion
Tuber brumale has often been considered as a common contaminant in commercial truffières throughout Europe ([29]). The competitive interactions between T. brumale and the other valuable black truffles were usually studied by targeting ECMs ([6]) and, to a lesser extent, soil mycelium ([4]). Here, for the first time, we evaluated the spatial distribution of T. brumale against both T. aestivum and T. melanosporum in the same experimental site and cultural conditions, by targeting either their ECMs or ERM.
In general, the ERMs of the three target species were more diffuse than their respective ECMs, a trend particularly evident for T. brumale. Moreover, different truffle ERMs co-occurred in a number of soil cores much higher than the respective ECMs.
Fourteen years after the establishment of the investigated orchard, the competition for space among the three black truffle species seems to be almost confined to the transect areas. Tuber aestivum and T. melanosporum ECMs and ERM were only partially replaced in the transects and T. brumale does not appear to colonize the other truffle plots. This consideration is also supported by the distribution of the brûlés in the truffle plantation. These characteristic areas devoid of vegetation are much more evident in T. aestivum and T. melanosporum than T. brumale growth sites ([31]). In our plantation, brûlés were completely absent within T. brumale plot and rare within the transect areas, whereas they were visible around almost all the plants within T. aestivum and T. melanosporum plots. Further confirmation of this hypothesis was obtained by the analyses of ECMs and ERM sampled within the T. melanosporum plot.
ECMs formed by other fungi were less abundant than those of the introduced truffles. The highest diversity and abundance of these fungi were found in the aest/brum transect, where three mature ectomycorrhizal trees at the edge of T. aestivum plot might have facilitated the colonization of truffle plants. This method of colonization by native ectomycorrhizal fungi has been discussed by several authors ([40], [11]), who consider native host trees as one of the first issues to solve when a new truffle plantation has to be established.
Competition between T. brumale and T. aestivum has been only poorly investigated. ECMs of T. brumale have been found in T. aestivum plants ([2], [42], [6]) but specific studies supporting the possibility that these species can significantly replace each other in cultural or natural conditions have never been carried out. In our study site, ECMs and ERM of T. brumale showed a more even distribution than those of T. aestivum that, conversely, dominated a single soil patch in the centre of the aest/brum transect. The lack of T. aestivum ECMs and ERM in half the plant rows could be due to the gradual soil and root colonization of T. brumale or, more likely, of native ectomycorrhizal fungal species. Tuber aestivum ECMs and ERM were also found in three soil samples in the brum/ mela transect. In this case too, T. aestivum dominated a soil patch (smaller than the previous one) where only T. brumale co-occurred both as ECMs and ERM. These truffle species seem to adopt two opposite ecological strategies. As a matter of fact, T. aestivum appears to be less efficient in soil exploration than T. brumale but its presence strongly reduces the ectomycorrhizal fungal diversity, as also reported by other authors ([39], [4]). The presence of T. aestivum in the brum/ mela transect may be explained as the consequence of a cross contamination during nursery mycorrhization ([19], [26]) rather than a mycelium or spore movement from the aest/ brum transect.
Tuber brumale has been ranked by several authors as a damaging species for truffle plantations ([27]), able to displace T. melanosporum and reduce its productivity ([10], [9], [25], [35], [34], [38], [31]). It is considered so threatening that cultural practices in T. melanosporum plantations in France are mostly devoted to reducing its presence in the soil ([40], [31]).
In this study, T. brumale was never found invading T. melanosporum, although in some plant rows its ECMs expanded up to the sampling points closest to T. melanosporum plants. Tuber brumale ERM was found in two cores collected in the sampling points beyond the T. melanosporum plants, mixed together with T. melanosporum ERM. At the same time, however, T. melanosporum is still well represented both as ECMs and ERM inside the transect.
Competition between black truffles mostly depends on a number of edaphic factors, such as organic matter content and soil pH. In the truffle plantation under investigation, the organic matter percentage is pretty low (1.2%) and pH is > 7.5, conditions that could have prevented T. brumale from colonizing the T. melanosporum plot. Several authors ([22], [9]) claim that a low level of organic matter favours T. melanosporum rather than T. brumale. Moreover, T. melanosporum has a better development in alkaline soils with pH that ranges between 7.5 and 8.3 ([22]), while the optimal pH for T. brumale is 6.5 ([8]). The host species may also promote the competitiveness of one or the other Tuber species even if, in this case, the results in literature are more ambiguous. Some studies reveal that T. melanosporum proved to be more competitive on Quercus spp., contrary to T. brumale which prefers C. avellana ([11], [14], [3]). Another condition that might have also prevented the diffusion of T. brumale is the no-tillage condition adopted in the truffle plantation. In fact, soil tillage is considered able to favour the propagation of Tuber competing species ([11]).
Conclusions
In this study site, T. brumale was not able to spread out into the T. aestivum and T. melanosporum plots and the competition seemed to be confined to the transect areas. When the selection of the plantation site is appropriate for T. aestivum and T. melanosporum, the main issue is the risk of nursery contamination, the primary cause of subsequent contaminations in the field ([26]). It is therefore crucial to carefully monitor the quality of both the spore inoculum and the Tuber mycorrhized plants before the establishment of the plantation. The use of mycelial inoculated plants, which recently proved to be successful with T. borchii cultivation ([20]), could also be a valid solution because mycelium has several advantages over spore inoculum, such as fewer contamination risks and higher percentages of root colonization.
Aknowledgements
The authors would like to thank Gianluigi Gregori and Danilo Tognetti of ASSAM (Agenzia Servizi Settore Agroalimentare Marche, Osimo, AN, Italy) for providing climate data of the study area and Dr. Susan West (Arancho Doc, translation and Localization Services) for revising the English style of the manuscript.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Enrico Stagnini
Mirco Iotti
Department of Life, Health and Environmental Sciences, University of L’Aquila, I-67100 L’Aquila (Italy)
Valentina Balestrini
Alessandra Zambonelli
Department of Agricultural and Food Sciences, University of Bologna, v.le Fanin 46, I-40127 Bologna (Italy)
Corresponding author
Paper Info
Citation
Ori F, Leonardi P, Stagnini E, Balestrini V, Iotti M, Zambonelli A (2018). Is Tuber brumale a threat to T. melanosporum and T. aestivum plantations?. iForest 11: 775-780. - doi: 10.3832/ifor2785-011
Academic Editor
Alberto Santini
Paper history
Received: Mar 13, 2018
Accepted: Sep 05, 2018
First online: Nov 28, 2018
Publication Date: Dec 31, 2018
Publication Time: 2.80 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 34252
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 29661
Abstract Page Views: 1691
PDF Downloads: 2258
Citation/Reference Downloads: 10
XML Downloads: 632
Web Metrics
Days since publication: 1970
Overall contacts: 34252
Avg. contacts per week: 121.71
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Feb 2023)
Total number of cites (since 2018): 3
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of black locust and black pine on extremely degraded sites 60 years after afforestation - a case study of the Grdelica Gorge (southeastern Serbia)
vol. 9, pp. 235-243 (online: 22 August 2015)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Short Communications
Detection and quantification of the air inoculum of Caliciopsis pinea in a plantation of Pinus radiata in Italy
vol. 12, pp. 193-198 (online: 10 April 2019)
Research Articles
Allometric equations to assess biomass, carbon and nitrogen content of black pine and red pine trees in southern Korea
vol. 10, pp. 483-490 (online: 12 April 2017)
Research Articles
Species interactions in pure and mixed-species stands of silver fir and European beech in Mediterranean mountains
vol. 14, pp. 1-11 (online: 02 January 2021)
Technical Notes
Effect of tree age on chemical compounds of ancient Anatolian black pine (Pinus nigra subsp. pallasiana) needles in Northwest Turkey
vol. 11, pp. 406-410 (online: 15 May 2018)
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
Research Articles
Verticillium wilt of Ailanthus altissima in Italy caused by V. dahliae: new outbreaks from Tuscany
vol. 13, pp. 238-245 (online: 19 June 2020)
Research Articles
Understory vegetation dynamics and tree regeneration as affected by deer herbivory in temperate hardwood forests
vol. 10, pp. 837-844 (online: 26 October 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword