Detection and quantification of the air inoculum of Caliciopsis pinea in a plantation of Pinus radiata in Italy
iForest - Biogeosciences and Forestry, Volume 12, Issue 2, Pages 193-198 (2019)
doi: https://doi.org/10.3832/ifor2866-012
Published: Apr 10, 2019 - Copyright © 2019 SISEF
Short Communications
Abstract
Caliciopsis pinea has been historically described as a secondary pathogen of pines. However, it has recently been associated with severe damages on Pinus radiata in Italy. Our study focused on the description of the seasonal spore dispersal of C. pinea and its relation to meteorological conditions (temperature, leaf wetness, relative humidity and precipitations). For this experiment one infected P. radiata plantation was sampled in Tuscany (Italy). A rotating arm spore trap together with a weather station were installed to sample the aerospora for 24 h every week from May to November 2016. Exposed tapes from spore traps were directly analyzed after DNA extraction by qPCR using specific primers and TaqMan MGB probe. The study shows an irregular occurrence of the inoculum of C. pinea throughout the whole sampling period with peak levels in mid-June and early August. The statistical analysis of the DNA and climatic data clearly show the strong influence of precipitation on the spore production of this pathogen. Furthermore, the very low detection limit of the qPCR experiment shows the efficacy and suitability of rotating arm spore traps for early detection of this pathogen.
Keywords
Introduction
Pinus radiata D. Don represents one of the most successful forest plantation species worldwide. This species has been planted in many parts of the world, such as Australia, Chile, New Zealand, South Africa and others ([23], [19], [32]). Currently, it represents the most widely planted conifer. Because of its fast development, profitable lumber and pulp production, about 25.000 ha of P. radiata were planted in Sardinia and in the peninsular Italy between 1960 and 1980 ([8]). As a consequence of forest fires and the effects of climate change in the Mediterranean region, P. radiata has been suffering a progressive decline in Italy over the last years. In addition, the sensitivity to frost, the lack of moisture and precipitation during the summer season, and the need for deep rich and well-drained soils also contributed to its collapse ([8]).
Additionally, some pests and diseases may represent a serious threat for P. radiata plantations. Among them, Caliciopsis pinea Peck, an ascomycetous fungus belonging to the family Coryneliaceae and order Coryneliales ([13]). It infects pine trees of all ages mainly in sandy and well-drained soils in North America ([25]). In eastern USA C. pinea may affect Pinus echinata Mill, Pinus pungens Lamb, Pinus rigida Mill, Pinus virginiana Mill, Pinus taeda L. and Pinus strobus L., the white pine, which is suffering a severe damage over the last years ([17], [24]). C. pinea has been observed in Europe on exotic pine species, such as Pinus pumila (Pall.) Regel ([30]), P. echinata, P. pungens, P. rigida, P. virginiana and P. radiata ([3], [20]), and on native European pines, such as, Pinus pinaster Ait., Pinus pinea L. and Pinus halepensis Mill ([18], [10]). Particularly, in Italy, it was firstly described as causing cortical cankers in young plantations of P. pinaster and Pinus insignis Dougl. (= P. radiata D. Don) ([3]). The pathogen has been also reported on Pinus nigra Dougl. ([4]).
Symptoms caused by C. pinea infection include sharply delimited cankers on trunks and branches crown wilting, defoliation ([29]) and a profuse resin production. Previous studies showed that the cankers did not produce significant damage ([29], [9]), but other authors have associated the presence of C. pinea with top killing cankers on trees ([17]). C. pinea produces small, globose, clustered, black pycnidia and stalked perithecia that arise from a stromatic cushion ([22], [14]). Black-hair-like fruiting structures persist throughout the year and spores mature in late winter and spring. Spores are disseminated by wind and rain and typically enter through bark lenticels or small insect wounds ([15], [10]).
The airborne inoculum of forest pathogens is generally underestimated in epidemiological studies. In particular, no information on C. pinea sporulation and its relationship with environmental parameters have been studied so far. However, due to the climate changes in the Mediterranean region, the pathogen sporulation and dispersal may be favored, leading to an increase of the disease incidence ([31]). As nowadays new spore trapping tools coupled with sensitive molecular methods give the opportunity to efficiently collect and detect airborne fungal inoculum ([2]), here we propose to analyze the seasonal spore dispersal of C. pinea in a P. radiata plantation, and its relation to local climatic conditions by using a rotating-arm spore trapping method combined with qPCR.
Materials and methods
Sampling area
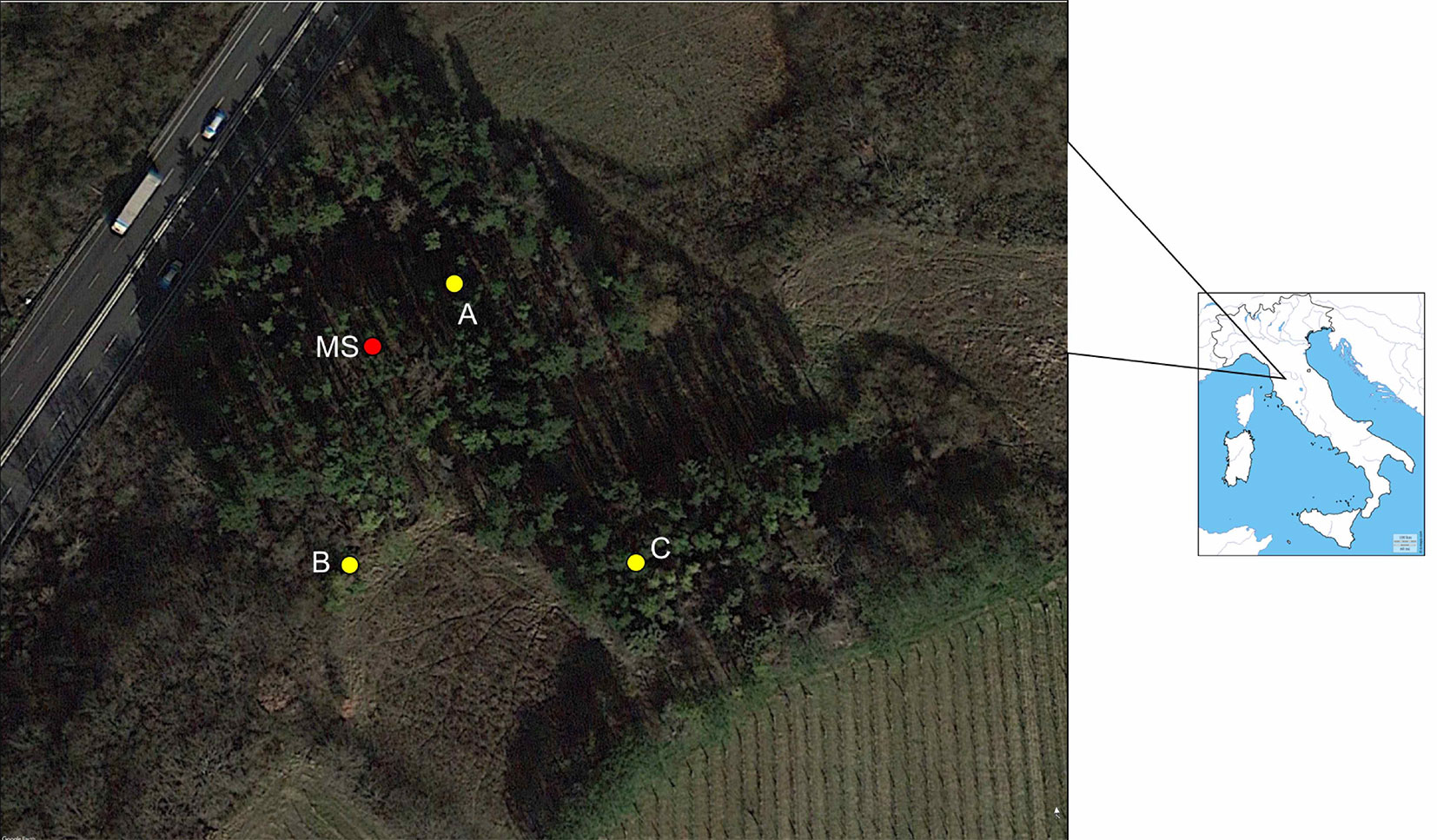
A P. radiata plantation in Carcheri (Lastra a Signa, Florence, Italy - 43.715875° N, 11.073649° E) was selected for the aim of this study. The stand is a 2-ha monospecific plantation of P. radiata, in a northwest facing slope (Fig. 1). The undergrowth is characterized by some Mediterranean shrub species, including some oaks (Quercus pubescens, Q. cerris) and other native tree species (Acer campestris, Fraxinus ornus, Rosa canina, Cornus mas, Sorbus estica, Crataegus monogyna, Equisetum sp. and Asparagus officinalis).
Fig. 1 - Location of the spore traps (A, B, C) and the meteo station (MS) at the sampling site in Carcheri (Tuscany, central Italy) .
The plantation showed 44.7% of trees affected by Caliciopsis cankers with abundance of resin and characteristic fruit bodies from the lesion (Fig. 2a, Fig. 2b). Cankers length ranged around 1-3 cm. The pathogen was detected by isolation on PDA ([20]) and molecular techniques ([21]).
Fig. 2 - (a) Caliciopsis cankers on a P. radiata trunk; (b) Black-hair-like fruiting bodies of Caliciopsis pinea growing from a canker; (c) RAST device; (d) meteorological station.
Spore trapping and climate data
The rotating-arm spore traps ROTTRAP 120 (RAST - Fig. 2c) were constructed by Milon Dvorák (Boršov nad Vltavou, Czech Republic), based on the principles of Perkins & Leighton ([27]), developed by Chandelier et al. ([7]) and modified by Dvorák et al. ([12]). An electric motor rotates 2400 rpm with a 0.8 mm thick U-shaped, square section wire. The vertical impactors of 50 mm in length distanced at 200 mm were covered for every sampling with a new double-sided non-woven tape (Tesa SE®, Norderstadt, Germany). Covering the front side (according to the direction of rotation) of each impactor, the spore trap provided an impaction area of 80 mm2. RASTs were sampling the air 1.4 m above the ground at a speed of 120 L min-1 with almost a 100% collecting efficiency for particles bigger than 7.18 μm. This limiting spherical diameter of particles was calculated using equations and charts published by Noll ([26]). In his paper a comprehensive theoretical and experimental testing of rotating-arm spore traps with variable parameters was reported.
The RASTs were placed in three spots (A, B and C - Fig. 1) of the infested forest at a distance of about 100 m each other. The automatic weather station (AMET, Velké Bílovice, Czech Republic) was installed next to the RAST on the spot A (Fig. 1, Fig. 2d).
The RASTs were sampling 24 consecutive hours once a week for a period of 29 continuous weeks from the May 17th to November 29th, 2016. After each sampling period, the exposed tapes were immediately placed into 2-ml sterile microtubes and stored at -20 °C.
The automatic weather station (Fig. 2d) carried out climatic measurements continuously over the course of the entire sampling period. Variables were recorded every 10 minutes and included air temperature, air relative humidity, dew point, leaf wetness and precipitations. The mean daily temperature, as well as other mean daily values, were calculated as an average of these measurements from midnight to midnight.
DNA extraction from the spore trap samples
DNA extractions included negative controls, which were prepared as microtubes with no tape rods.
For the purpose of spore disruption, we added 0.3 g of 0.1 mm and 0.1 g of 0.5 mm balotina beads and 250 µl of 0.1% Nonidet P40®-substitute (AppliChem, Darmstadt, Germany) to each tube. The disruption itself was then accomplished by a Mixer Mill MM400® (Retsch, Haan, Germany) activated for 10 min at 30 Hz. Then, the lysis buffer ([11]) and 4 µl RNase A (E.Z.N.A.® Plant DNA kit, Omega Bio-tek, Norcross, GA, USA) were added to all samples. The mixture was incubated for 60 min at 65 °C with occasional mixing. For further processing, the E.Z.N.A. Plant Mini Kit was used. The elution used 100 µl of preheated buffer after 10 min of incubation at room temperature ([11]).
qPCR experiments
The primer set Cpin-F (5’-ATTGTCGTCTGAATTCTGATGCA-3’) and Cpin-R (5’-TTCATCGATGCCAGAACCAA-3’) and the TaqMan® MGB probe (Cpin-Pr) (5’-VIC-AATAAAACTTTCAACAACGGATC-MGBNFQ-3’) described by Luchi et al. ([21]) were used to detect and quantify C. pinea by real-time quantitative PCR (qPCR).
DNA extracts from spore trap samples were assayed in MicroAmp Fast 96-well Reaction Plate (0.1 mL) closed with Optical Adhesive, and by using the StepOnePlusTM Real Time PCR System (Applied Biosystems, Life Science, Foster City, CA, USA). PCR reaction was performed in a 25 μl final volume containing: 12.5 μl TaqMan Universal master mix (Applied Biosystems), 300 nM forward primer (Eurofins MWG Operon, Ebersberg, Germany), 300nM reverse primer (Eurofins MWG Operon, USA), and 200 nM TaqMan MGB probe (Applied Biosystems), 20 ng genomic DNA. Each DNA sample was assayed in duplicate. Two wells, each containing 5μl of sterile water, were used as the no-template control (NTC). Additionally, two wells containing DNA extracted from infected C. pinea pine tissue (positive control), and two wells containing DNA from healthy pine tissues (negative control - [21]) were included. The PCR protocol was 50 °C (2 min), 95 °C (10 min), 50 cycles of 95 °C (30 s), and 60 °C (1 min). Results were analyzed by using a SDS 1.9 Sequence Detection System (Applied Biosystems) after manual adjustment of the baseline and fluorescence threshold. The standard curve for the absolute quantification of C. pinea DNA was generated with 5-fold serial dilutions (ranging from 25 ng/tube to 40 pg/tube) of known concentration of DNA of C. pinea (strain IT1, IPSP-CNR collection - [21]). The amount of fungal DNA from spore trap samples was expressed as pg DNA C. pinea / µg of total DNA extracted.
Data analysis
Differences in C. pinea DNA quantity among different spore traps and sampling periods were tested by the analysis of variance (ANOVA) followed by Tukey’s HSD post-hoc test.
Influence of climatic conditions on the occurrence of C. pinea inoculum was evaluated by the Random Forest algorithm utilizing conditional inference trees as base learners provided in the “party” package. Variable selection was done by the permutation-based attribute selection algorithm provided in the “Boruta” package. The conditional computation of variable importance accounting for correlated predictors implemented in the “party” package was used as a function to obtain feature importance. The importance of variables that were deemed important by the selection algorithm was then expressed by means of permutation-based mean decrease in accuracy (%IncMSE) using the function “varimp”, which calculates the increase in mean squared error (MSE) of predictions when the considered variable is permuted by random shuffling.
The set of predictors that were used as input for the feature importance procedure included values of mean daily temperature, relative humidity, leaf wetness and daily precipitation during the sampling day and every particular day of 40 days before the sampling.
All analyses were carried out by using the R software ([28]).
Results and discussion
Occurrence of the air inoculum of C. pinea
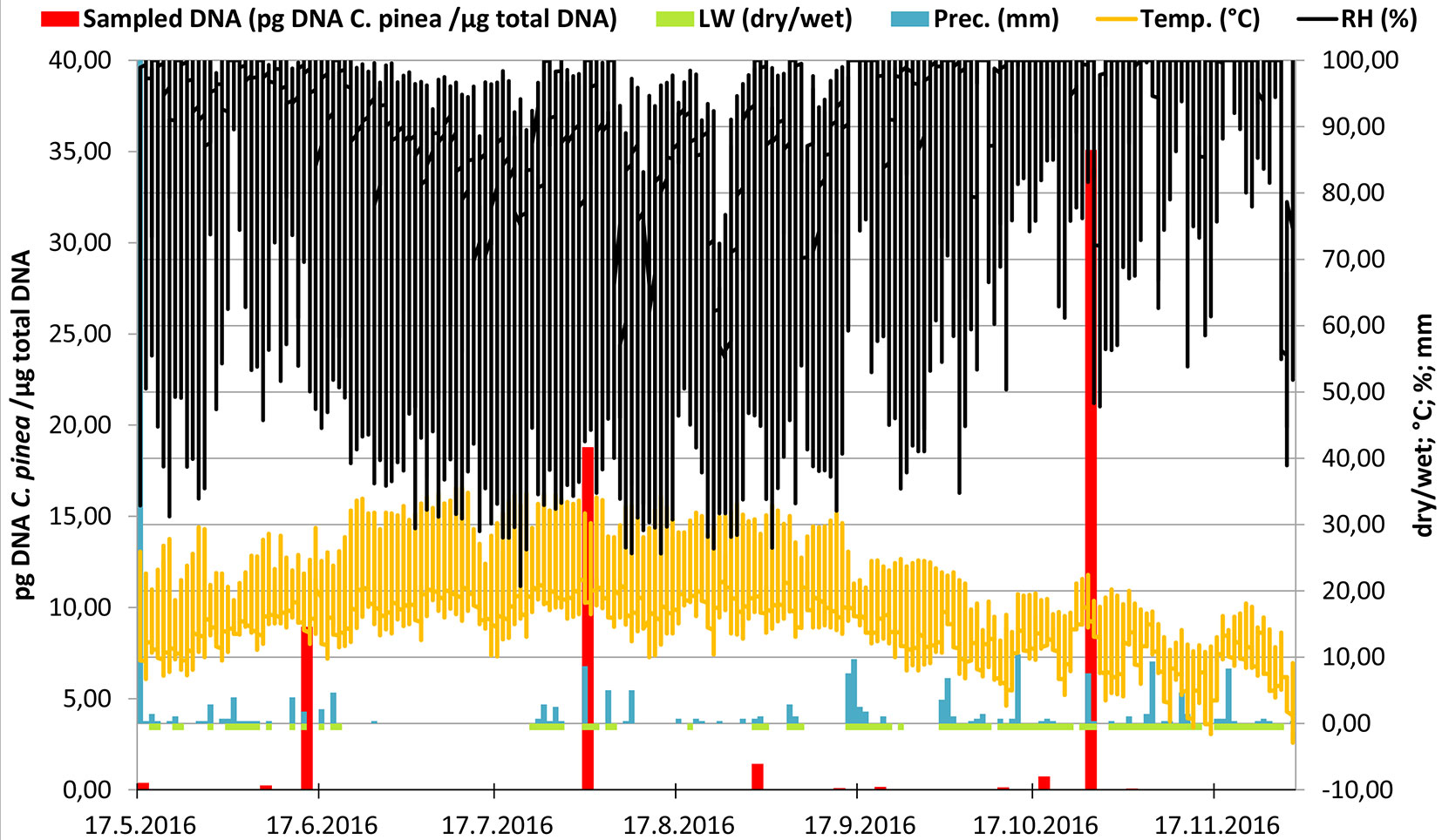
Our study shows that C. pinea inoculum is present in aerobiological samples collected in a P. radiata plantation from May to November 2016 (throughout the entire sampling period). No significant differences (p = 0.07) of C. pinea DNA collected among the traps placed in three different spots (A, B, C) were observed. A continuous C. pinea inoculum production has been recorded over the whole sampling period (May-November). Despite no significant differences (p = 0.824) were observed among different months, three outstanding peaks of spore production were recorded (mid-June, early August and late October - Fig. 3).
Fig. 3 - Representation of the meteorological variables in relation with the amount of airborne inoculum of C. pinea detected over the whole sampling period. The x-axis shows the progress of time. Left y-axis displays the template DNA quantity (pg) detected in 1 μg of total extracted DNA in a logarithmic scale and reflects the amount of C. pinea inoculum trapped by the rotating arm spore trap (red columns). Daily range of precipitation (“Prec.”: light blue bars, mm per day), temperature (“Temp.”: orange line, °C), leaf wetness (“LW”: green line, scaleless; 1 = dry, 0 = absolutely wet) and relative humidity (“RH”, black line, %) are shown on an average daily basis and scaled by the right y-axis.
Although C. pinea triggered damages on P. strobus in the 1930s and some epidemiological studies were conducted in North America ([29], [22]), it has arisen scientific attention only in 2009 as a result of unexpected serious damages on P. strobus forests throughout several north-eastern US states ([24], [25]). In Europe, it is even less studied since it was traditionally pointed as a secondary pathogen ([10]) related to stressed trees in poor plantations ([4]). C. pinea has been reported in Italy since several years as the causal agent of necrosis and cankers on stems and branches on P. pinea, P. nigra and P. radiata ([3], [4], [20]). A possible reason for the lack of knowledge on this pathogen is that the identification of Caliciopsis canker symptoms become more challenging as tree size increases ([24], [25]). Moreover, C. pinea symptoms, such as pitching blackened, rough, resinosis and sunken bark, are similar to (and may be confused with) those caused by other important pine pathogens like Fusarium circinatum, Diplodia pinea or Diplodia scrobiculata. The capability of C. pinea to play a combined role in fungal infections on pines has been observed in 2006 on P. strobus trees in Bath County, VA (USA), where C. pinea was isolated together with D. scrobiculata from cankers and lesions of branches and stems ([9]). Likewise, it was collected in 2010 from a P. radiata sample in Spain, where both C. pinea and F. circinatum occurred on the same stem ([5]). However, in our study F. circinatum was never detected from any of the conspicuous tissue explants made from symptomatic P. radiata tissues collected in the sampling area (data not shown). Conversely, in Carcheri the characteristic fruiting bodies of the C. pinea were observed on small cankers on primary and secondary branches of P. nigra twigs affected by D. pinea ([1]).
Influence of climatic conditions on the occurrence of C. pinea
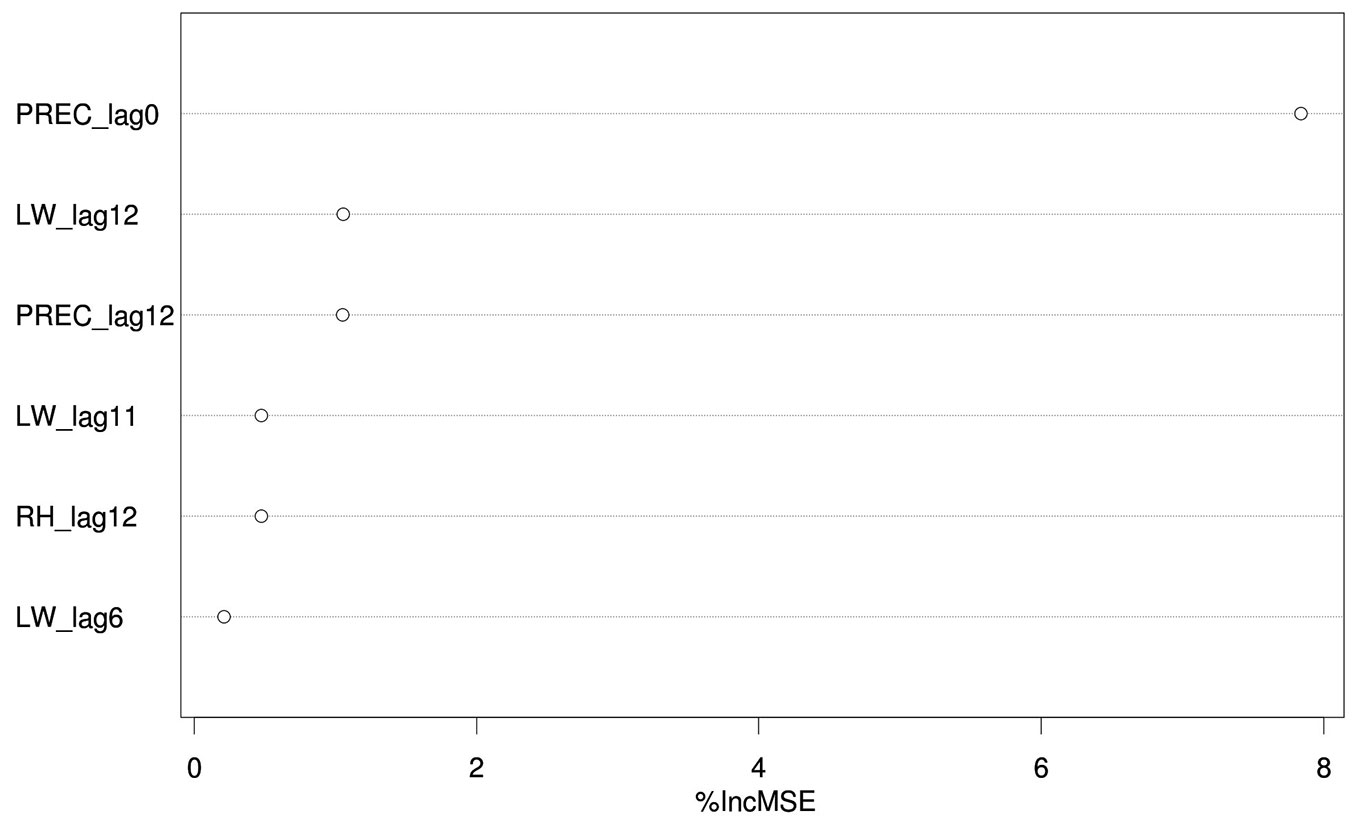
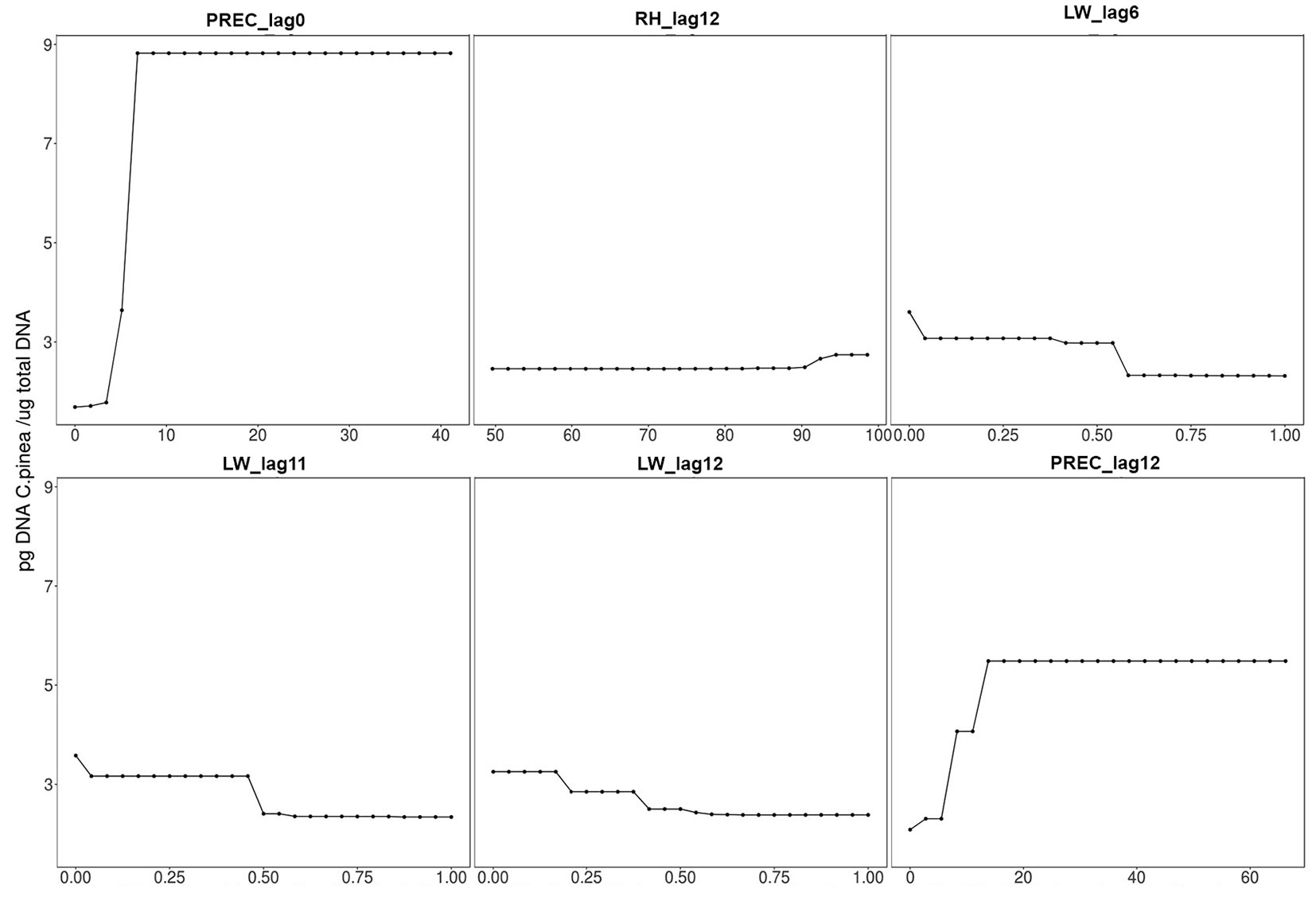
The load of C. pinea spores in the air proved to be mostly influenced by precipitation during the sampling day and, to a lesser extent, by the amount of precipitation, leaf wetness and relative humidity of the 12th day before sampling (i.e., spore trapping), leaf wetness of the 11th day before and relative humidity of the 6th day before. The importance of these variables (i.e., permutation-based mean decrease in accuracy) is shown in decreasing order in Fig. 4. All other variables were deemed not important by the algorithm used. Partial dependence plots, i.e., the dependence of C. pinea DNA amount on the significant attributes accounting for all other tested features, are displayed in Fig. 5. These results may be explained by considering the chain of events in terms of climatic conditions, fructification and sporulation. Indeed, climatic conditions may influence the formation of fruiting bodies, which has a direct impact on the number of spores produced, but they also affect sporulation, which may take place several days after the formation of fruiting bodies. This can explain the significant effect of both lagged (11-12 days before sampling) and non-lagged (like PREC_lag0) climatic variables on the Caliciopsis pinea air inoculum observed in this study.
Fig. 4 - Importance (%incMSE, permutation-based mean decrease in accuracy derived from the Random Forest algorithm) of predictors significantly accounting for the amount of airborne C. pinea spores. (PREC): precipitation (mm); (LW): leaf wetness (1=dry; 0=wet); (RH): relative humidity (%); (_lagX): Xth days before sampling.
Fig. 5 - Partial dependence plots for significant variables. (PREC): precipitation (mm); (LW): leaf wetness (1=dry; 0=wet); (RH): relative humidity (%); (_lagX): Xth days before sampling.
Other studies have pointed out the influence of precipitations on the inoculum occurrence of other forest pathogens, such as F. circinatum ([16]), Dothistroma septosporum ([33]) and Hymenoscyphus fraxineus ([6], [11]). In the latter case, a significantly higher concentration of spores was detected in wetter than in drier localities in the Czech Republic ([6]) and the highest correlation was found between relative humidity and H. fraxineus air inoculum records with an approximately 13-day lag ([6], [11]). In the present study the highest correlation (75.96%) of C. pinea air inoculum was found with the relative humidity recorded 11 days before sampling.
Efficiency of rotating arm spore traps and qPCR for the detection of C. pinea
The rotating arm spore traps combined with qPCR assay allowed an efficient and suitable method for early detection of this pathogen showing an extreme sensitivity up to 7.74 × 10-4 pg DNA C. pinea / µg of total DNA extracted on 11-12TH July, 2016 (Fig. 3). This type of spore trap has been previously utilized for the detection and quantification of the air inoculum of other important forest pathogens, such as H. fraxineus in Belgium ([7]) and the Czech Republic ([12]), and more recently for the detection of F. circinatum in a plantation of P. radiata in Galicia, Spain ([12]). Using the Noll’s equation ([26]), ROTTRAP 120 was calculated to be almost 100% efficient for particles of spherical diameter of at least 7.18 µm ([12]). The chiefly ellipsoidal ascospores of C. pinea are 5-6 × 3-3.5 µm ([13], [18]), and the spermatia 2.5-3.5 µm in length. Regarding the theoretical calculation of Noll ([26]), particles of such size are trapped by ROTTRAP 120 with the efficiency of 50 % and 13 %, respectively. Consequently, the detected inoculum of C. pinea might be strongly underestimated and the actual air-borne spores’ amount might be higher. The C. pinea inoculum was collected in 25 out of the 29 sampling days, demonstrating that the inoculum production of this pathogen covers a large part of the vegetative season.
Conclusions
The efficiency and suitability of rotating arm spore traps combined with the sensitivity of qPCR allowed the detection of C. pinea inoculum in the pine stand during the sampling period. The results here obtained improve our understanding of C. pinea epidemiology showing that the period of 12 days before the spore occurrence, which is influenced by higher relative humidity and precipitations, may enhance the fruiting body development. Later, immediate release of spores in mature fruiting bodies is triggered by rain. This approach could be applied for an efficient and effective management and control measures against this pathogen in pinewood stands.
Acknowledgements
We are thankful to the COST Action FP1406 “Strategies for management of Giberella circinata in greenhouses and forests” (PINESTRENGTH) for the support of a Short Scientific Mission “STSM” of Aneta Bacova at the IPSP-CNR (Sesto Fiorentino, Italy) during September 2016. The study was financed by the project LD15046: “Detection and biology of Gibberella circinata-essentials for early warning and management strategies in the Czech Republic” (Ministry of Education, Youth and Sports of the Czech Republic). The authors wish to thank Francesco Pecori and Paola Bartolini for their help in field sampling and lab assistance.
Author contributions
LB, MD and NL conceived of the study; AB, ALP, NL, MD and LB performed the experiments; TK and LB analysed the data; LB, MD, AS and NL wrote the manuscript.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Aneta Bačová
Tomáš Kudláček
Phytophthora Research Centre, Department of Forest Protection and Wildlife Management, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelská 1, 613 00 Brno (Czech Republic)
Department of Forest Protection and Wildlife Management, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelská 1, 613 00 Brno (Czech Republic)
Alberto Santini 0000-0002-7955-9207
Luisa Ghelardini 0000-0002-3180-4226
Nicola Luchi 0000-0003-3119-7574
Institute for Sustainable Plant Protection, National Research Council (IPSP-CNR), v. Madonna del Piano 10, 50019 Sesto Fiorentino, Firenze (Italy)
Corresponding author
Paper Info
Citation
Botella L, Bačová A, Dvorák M, Kudláček T, Pepori AL, Santini A, Ghelardini L, Luchi N (2019). Detection and quantification of the air inoculum of Caliciopsis pinea in a plantation of Pinus radiata in Italy. iForest 12: 193-198. - doi: 10.3832/ifor2866-012
Academic Editor
Claudia Cocozza
Paper history
Received: May 24, 2018
Accepted: Jan 21, 2019
First online: Apr 10, 2019
Publication Date: Apr 30, 2019
Publication Time: 2.63 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 33952
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 28948
Abstract Page Views: 2316
PDF Downloads: 2131
Citation/Reference Downloads: 6
XML Downloads: 551
Web Metrics
Days since publication: 1837
Overall contacts: 33952
Avg. contacts per week: 129.38
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Feb 2023)
Total number of cites (since 2019): 5
Average cites per year: 1.00
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Real-Time PCR for Ceratocystis platani detection: in-depth validation to assess the diagnostic potential and include additional technical options
vol. 11, pp. 499-509 (online: 18 July 2018)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Research Articles
Latent infection of Biscogniauxia nummularia in Fagus sylvatica: a possible bioindicator of beech health conditions
vol. 9, pp. 49-54 (online: 18 June 2015)
Research Articles
Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback
vol. 12, pp. 160-165 (online: 04 March 2019)
Research Articles
Soil water deficit as a tool to measure water stress and inform silvicultural management in the Cape Forest Regions, South Africa
vol. 13, pp. 473-481 (online: 01 November 2020)
Research Articles
Outcome of Ceratocystis platani inoculations in Platanus × acerifolia in relation to season and inoculum dose
vol. 9, pp. 608-617 (online: 17 March 2016)
Research Articles
Allometric equations to assess biomass, carbon and nitrogen content of black pine and red pine trees in southern Korea
vol. 10, pp. 483-490 (online: 12 April 2017)
Research Articles
Post-fire recovery of the plant community in Pinus brutia forests: active vs. indirect restoration techniques after salvage logging
vol. 11, pp. 635-642 (online: 04 October 2018)
Research Articles
Use of δ13C as water stress indicator and potential silvicultural decision support tool in Pinus radiata stand management in South Africa
vol. 12, pp. 51-60 (online: 24 January 2019)
Research Articles
Amount and distribution of coarse woody debris in pine ecosystems of north-western Spain, Russia and the United States
vol. 7, pp. 53-60 (online: 28 October 2013)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword